FUEL CELL
Information About Fuel Cells
In 1839 William Robert Grove invented the fuel cell -
the development has been slow. The fuel cell is an electrochemical device
as it makes use of the chemical energy of the fuel to generate electricity.
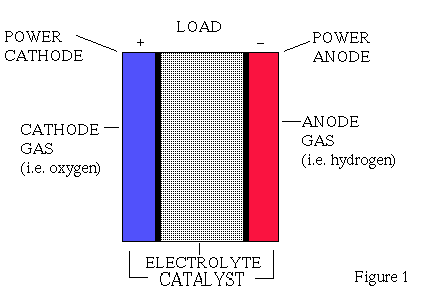
The most common type uses hydrogen and oxygen as fuel.The cell is divided
into three compartments (see Fig.1) .Two porous electrodes are separated
by an electrolyte, sulphuric acid. The cathode is fed with oxygen and the
anode with hydrogen. The gases penetrate into the electrolyte where they
form water and produce an electric current through an external circuit
connecting the two electrodes. A catalyst such as platinum is needed for
this reaction.
Advantages of Fuel Cells
- Fuel cells are not dependent on a single fuel source.
- Hydrogen-oxygen fuel cells use a renewable form of energy
as the product water can again be divided into hydrogen and oxygen e.g.
using solar power.
- Hydrogen-oxygen fuel cells release only water, electricity
and heat. Another fuel source is natural gas. Methanol could be converted
to a hydrogen rich gas that could then be used by the fuel cell. The emissions
are lower than with combustion turbines. Therefore the great advatage of
fuel cells is that they could help to reduce the air pollution.
- Fuel cells also have an advantage over batteries as they
can continue to convert energy as long as fuel is supplied.
Examples
Schematic Presentation of a Fuel Cell
Sources: The
Canadian Fuel Cell Homepage & The Energy Question by Gerald Foley
Back to CAR OF THE FUTURE