Secondary Battery Systems
Lead Acid, Nickel-Cadmium, Nickel Metal-Hybride,
Alkaline, Lithium.
Introduction
Modern technical systems such as portable computers, mobile telecommunication
sets and electrical cars need light and efficient batteries.
To increase capacity, self-discharge life and cycle life, computational
models and tests have to be developed. Also the testing methods in realistic
cells have to be accelerated. The availability of recycling technologies
should be the most important factor while developing new battery types,
as some batteries contain metals which pose a potential threat to our health
and environment.
More and more secondary battery systems are offered. But with increasing
capacity the cycle life was decreasing. Just the new Lithium cells have
better values, both in capacity and cycle life.
Capacity of rechargeable batteries (mAH)
|
NiCd |
NiMH |
Alkaline |
Lithium |
| Micro |
220 |
- |
- |
- |
| Mignon |
900 |
1200 |
1000 |
1350, 3.6V |
| Baby |
2800 |
2400 |
3000 |
- |
| Mono |
4000 |
4500 |
6000 |
- |
| E-Block |
110 |
120 |
- |
7000, 4.1V |
| Cycles |
600-900 |
300-500 |
100 |
>500 |
Summary for NiCd, NiMH and Alkaline
cells
Rechargeable Alkaline, NiMH and NiCd cells have nearly the same
power, so the life between charges is short, it's about 20%-40% of primary
Alkaline cells. The self-discharge rate cannot be ignored, it's about 1%/day
for NiCd and 2-3%/day for NiMH cells. For medium time storage they don't have
to be charged, as this only reduces battery life by one cycle. NiCd cells
offer the high-power that is required for HEV accelerations.
Summary for Lithium cells
Lithium batteries are very new. They have 3.0-5.0 volts, so they can't
be used with most standard equipment. Some new notebooks already use Lithium-Ion
batteries. Their self-discharge is less than 10% per month, that's very
low.
Summary for Lead Acid batteries
Lead Acid batteries are the best developed battery type, and therefore
the cheapest. Like NiCds, the offer high-power output.
Summary
Rechargeable batteries have a high initial cost, but they are the cheapest
over a long time period. For every battery system a special charger should
be used, to get the full cycle life. Cell life is also reduced by overcharging
or not fully discharching the cells, known as memory effect. On NiMH batteries
overcharging has a damaging effect. With every charge/discharge cycle
batteries lose a bit of their capacity. Here's the cycle life diagram
of a Li-ion battery by Maxell.
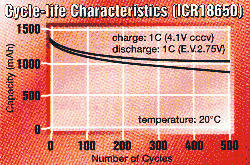
All cells should be used carefully to avoid possible hazards.
Do not let the battery come into contact with metals to avoid short circuiting,
as this may generate heat.
Do not deform the battery.
Do not connect or charge the battery with polarities reversed, as it may
explode.
Do not use different types of batteries together.
Stop using the battery if it leaks (it smells strange).
For more safety instructions goto Maxell.
Recommendation
Lithium cells may be used in future, but they are not available everywhere
up to now. We recommend using NiCd cells, as they provide the most power
per dollar. Only Lead Acid batteries are cheaper, but they are too big and
heavy to carry.
THE ALTERNATIVE ENERGY STORAGE GROUP:
Christoph Böhm TI4 TIW5CHBO@pcmail.rz.fht-esslingen.de
Gregor Lotti NT4 NTS5GRLO@rz.fht-esslingen.de
Patrick Lotti NT4 NTS5PALO@rz.fht-esslingen.de
