
Different Models of atoms 
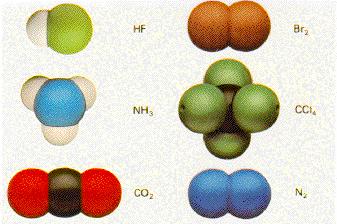
There are different models of an atomand its electrons.
First you can see models of different atoms which have a different number of electrons and here the atoms are combined with other atoms of a different element.
Here you can see why it is possible for an electron to orbit for example two atoms and their cores.
You also notice that the atoms are combined regularly. That means that there is a certain symmetry in their construction.
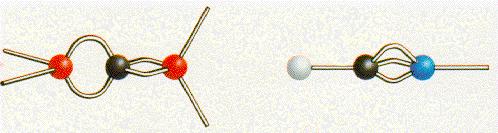 Another kind of
model is shown to you here :
Another kind of
model is shown to you here :
Here you can see the single connections between the single atoms and the connections which are free from atoms.
But not only the connections are shown, also the number of the connections are illustrated.
Another advantage is, that you know the number of the elctrons which are present and where they are placed.
So the structure of the combination of single atoms can bee seen here very clearly !
 Here you can
see a model of a certain material.
Here you can
see a model of a certain material.
As you can see they consist of more than one atom and there is more than one combination and more than one connection between the single atoms.
The structure of this model is given by the connection of the atoms which are combined with atoms of other elements.
This structure gives certain qualities to the material.
If the atoms were placed another way, then the material would have had other qualities. The look, the colour, the surface and the handling would be different.
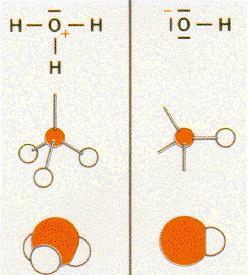 Two
examples for you :
Two
examples for you : 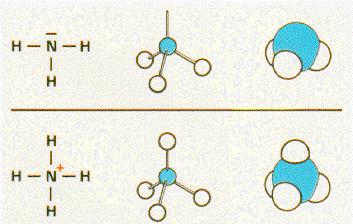
Here you can see again the different between the two possibilities to show you a model of an atom or a model of a combination of more then one atom.
Here you can also see how the structure of the model is created step by step.
But now let's go back to the atoms.
Copyright 1986 : Pictures taken from the book"elemente - Chemie I", Ernst Klett Verlage GmbH & Co. KG, Stuttgart 1986