

Everything around us is made of material. And all material is made of small atoms. But an atom is so small that you cannot see it with your eyes.
But here you can see a modell of an atom :
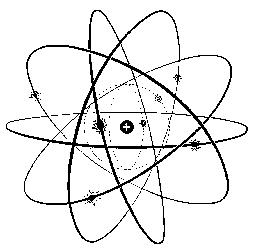
Today we know that an atom has a core which has a positive charge.
This core is sourrounded by electrons which have a negative charge.
The atomic core is made of positive protons and neutrons. Neutrons have got no charge, neither positive nor negative.
An atom is able to have more than one proton. But the number of the positive protons is equal to the number of the negative electrons.
So that is the reason why an atom is also nuteral.
The elctrons ly round the core of the atom at a very high speed at different distances above the core. You can compare that model with our earth and its satellites flying around our planet.
The satellites also have different distances to the earth and so they orbit the earth at different speeds.
Until now we have just looked at one single atom. But we just said, that the materials and elements are built up by more than one atom.
Copper for example is an element and it is also a conductor. That means that copper is able to conduct electric current .
Let us imagine that the atoms are very tiny bowls. Then an element would be made of small bowls, which are built up in a certain way.
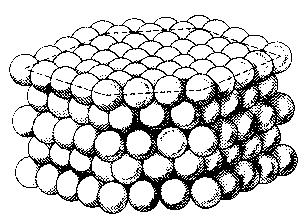
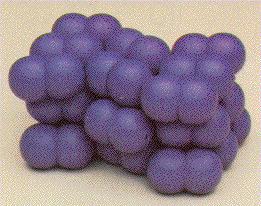
So you can see that the elements and materials are constructed just like a crystal.
You also call this kind of construction a crystal lattice.
You can see that every atom has got another atom next to it. So, if the atoms so close to each other, then the electrons also will have a very small distance to each other.
So it is possible that one electron which is closer to another atom core moves towards the other atom.
I know that this is hard to believe, but why don't you just try one little experiment with me and you will see that the information I just gave you is true.
And if the elctrons move from one atom to another in a certain direction,
then you can call it an electric current.
But not all the elements and the materials are conductors. You call an element only a conductor if their electrons are able to move from one atom to another.
But how can we "tell" the electrons to move and to change the atom ?
Well, we know by now that an electron is negatively charged and a proton is positively charged.
Because of this charge the electrons and the protons have got an electric
force :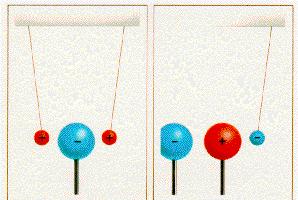
That means that a negative electron attracts a positive proton ! So if they are next to each other they will appoach each other.
Two charges of the same polarity repel each other. Increasing the distance, the electrons will move away from each other if they approach.
Now we can understand the experiment we did a couple of minutes ago !
An example of such an electric force is a battery.
The battery is made out of single cells. Each cell has got a certain voltage and together they have got a higher voltage than a single cell.
The battery has got a positive and a negative pole.
So the battery with its electric force and its voltage is able to push the electrons in the elements (which must be conductors) away.
To do this the voltage of the battery must be high enough so that the electric force of the battery is high enough to push the electrons and to make them move.
This phenomenon will be our next topic. So let´s see whats going on with the batteries.
Click here to return to the first page.
Copyright 1986: Pictures taken from the book "KOSMOS - Experimentierbuch für X 3000", Franckh'sche Verlagshandlung, W. Keller & Co., Stuttgart 1986
Copyright 1986 : Pictures taken from the book"elemente - Chemie I", Ernst Klett Verlage GmbH & Co. KG, Stuttgart 1986